Founded in 1992, Materia Medica Holding, a research and production company, developed its first original medications and launched their mass production quite soon after its establishment.
Video about the Company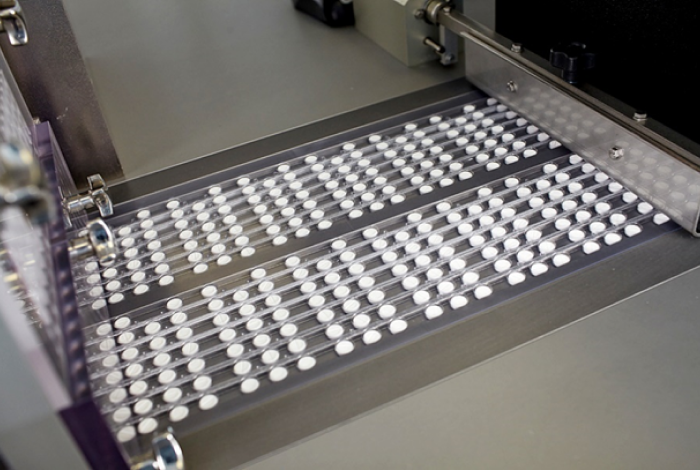
The Company’s products quickly gained the trust of doctors and patients.

The Company conducts intensive multicenter clinical trials and develops a new class of medications based on ultralow doses of antibodies to endogenous regulators.

The drug is intended for the treatment of alcohol addiction.

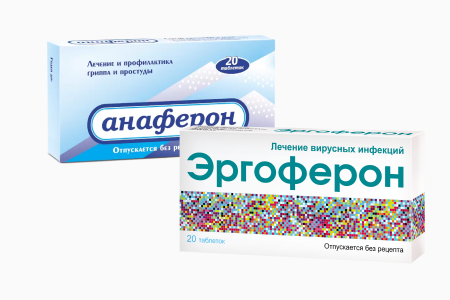
Both of these two medications are designed to treat and prevent flu and acute respiratory viral infections. Anaferon for Children is designed specifically for young children.



The prize was awarded for the development of a new class of medications based on ultralow doses of antibodies.


The factory is equipped with the most advanced manufacturing equipment of the world’s leading firms, which helps standardize the full cycle of the production of solid dosage forms.
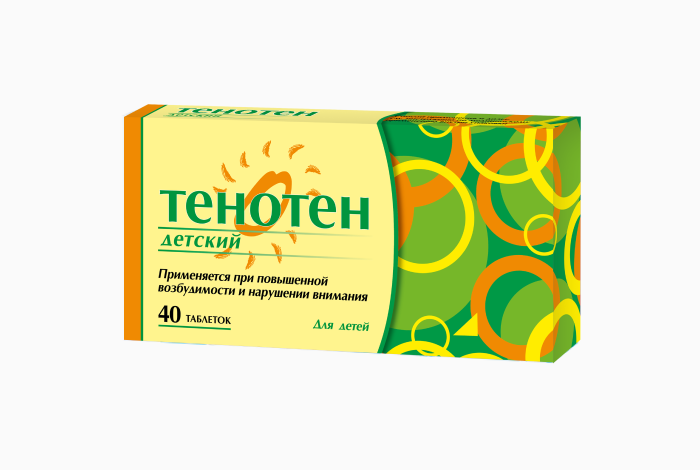
Tenoten for Children is the only sedative medicine created specifically for young children, taking into account the peculiarities of a child’s body.


The Federal Service for Surveillance in Healthcare and Social Development of the Russian Federation acknowledges that the manufacturing and quality control of medications by the Company are compliant with the requirements of OST 42-510-98 “Good Manufacturing Practice and Quality Control Rules for Medicinal Products (GMP)” and GOST R 52249-2009 “Good Manufacturing Practice and Quality Control Rules for Medicinal Products.”

The drug has a complex mechanism of action and is used for the treatment of acute respiratory viral infections and influenza.

Anaferon for Children is recognized as the most frequently prescribed medicine for children*.
* According to COMCON-Pharma data for 2012.

The Company passes two EU GMP audits and receives two GMP certificates.

The Anaferon brand is recognized as Brand of the Year No. 1 in Russia in the “Antiviral medications” category.
The Company becomes one of the leaders of the clinical trial market among Russian companies**.
** According to Synergy Research Group’s data for Q1 2013.


Yet again, the Anaferon brand becomes the winner of the Brand of the Year No. 1 prize in Russia, winning in the “Antiviral medications” category.
The Company opens a shop at its factory with a nominal capacity of up to one billion pills per year.

The drug is designed to address major male urological problems.

It is a third time that the Anaferon brand wins the Brand of the Year No. 1 prize in Russia, in the “Antiviral medications” category.

The Company is awarded the Best Employer status within the framework of the AON Best Employers Study 2017, a survey founded by AON Hewitt, a global HR consulting leader that conducts research in more than 90 countries worldwide.

Materia Medica Holding receives Platinum Ounce, one of the most prestigious awards of the Russian pharmaceutical community, in the category “Development of innovative medicines and their introduction into medical practice.”

Rengalin is recognized as Brand of the Year No. 1 in the category of anticough medicines.

Rengalin receives Green Cross, a professional pharmacy award, winning in the “Best anticough medicine” category.


According to a Synergy Research report on clinical trials in Russia in Q3 2018, Materia Medica Holding, which initiated four new clinical trials (with 1,388 patients) in Q3 2018, was ahead of all domestic participants accounted for in the ranking in terms of the number of clinical trials. Moreover, the Company outranked international pharma manufacturers in the number of trial subjects.

An international Russia—EU conference, “The Phenomenon of Release Activity: Prospects for Application in Medicine and Technology,” was held at the European Parliament building in Brussels. Leading experts from Russia, the U.S. and Europe confirmed the efficacy of medications based on ultrahigh dilutions for the treatment and prevention of various diseases.

Materia Medica Holding receives a certificate of compliance with GMP requirements in the manufacturing of veterinary products.

The medication Afalaza receives Green Cross, beating out competitors among “Medications of choice for the early treatment of benign prostatic hyperplasia”.

Ergoferon becomes Brand of the Year No. 1 in the category of antiviral medications.

At the 20th anniversary summit of HR directors of Russia and the CIS, Materia Medica Holding became the winner in the Talent Management System nomination, receiving the Crystal Pyramid 2019 Grand Prix for achievements in the field of human capital management.

The medication Afalaza wins the Green Cross award again, beating out competitors among “Medications of choice for the early treatment of benign prostate hyperplasia.”
Ergoferon wins the Green Cross pharmacy award in the category “Medication of choice for the treatment of colds and flu”.

Rengalin is awarded the Brand of the Year No. 1 prize as the best anticough medicine.

The Anaferon brand wins the annual Product of the Year 2020 award in the “Consumer Confidence” nomination of the “Immunomodulators” category.

Raphamin is a one-of-a-kind medicine that affects the molecules of the major histocompatibility complex (MHC), Class I and II. The MHC molecules are responsible for the recognition of viral and bacterial pathogens and participate in the activation of the immune response. As a result, Raphamin has not only an antiviral but also a moderately pronounced immune-mediated antibacterial effect. In the course of our research, a unique feature of the medication was experimentally proven: when Raphamin is used together with antibacterial medications, the efficacy of the antibiotic is enhanced even in case of initial resistance by pathogenic bacteria.
The developed combination of the Raphamin components is a fundamentally new solution in the treatment of acute respiratory tract infections.

Ergoferon wins in the category “Best antiviral medication.”
Tenoten is recognized as “The gold standard of therapy to achieve emotional tranquility.”

Ergoferon wins in the category “Best remedy for colds and influenza.”
Rengalin wins in the category “Best anticough therapy.”

Rengalin wins the “Consumer Confidence” nomination in the category “Products used in the treatment of dry and wet coughs.”
Ergoferon is acclaimed as the best “Choice of specialists and consumers” in the category “Antiviral medications for acute respiratory infections and influenza.”

Impaza is crowned as the best remedy in the category “Medication for the treatment of erectile dysfunction”.



